Who is looking for an ebola vaccine?
The number of studies listed on clinicaltrials.gov for ebola, 25, is less than for the fitbit, 26. This came as a surprise and led to further review of both sets of trials.
The set of studies targeting the ebola virus share the follow characteristics:
- The majority of ebola studies were started in the past year (2014).
- A majority of ebola trials have been sponsored by the US Government.
- The most active sponsors have only become active in the past year.
- Nearly all studies are Phase I studies.
1. The majority of ebola studies were started in the past year (2014).
16 out of the 25 studies listed were started in the past year (2014).
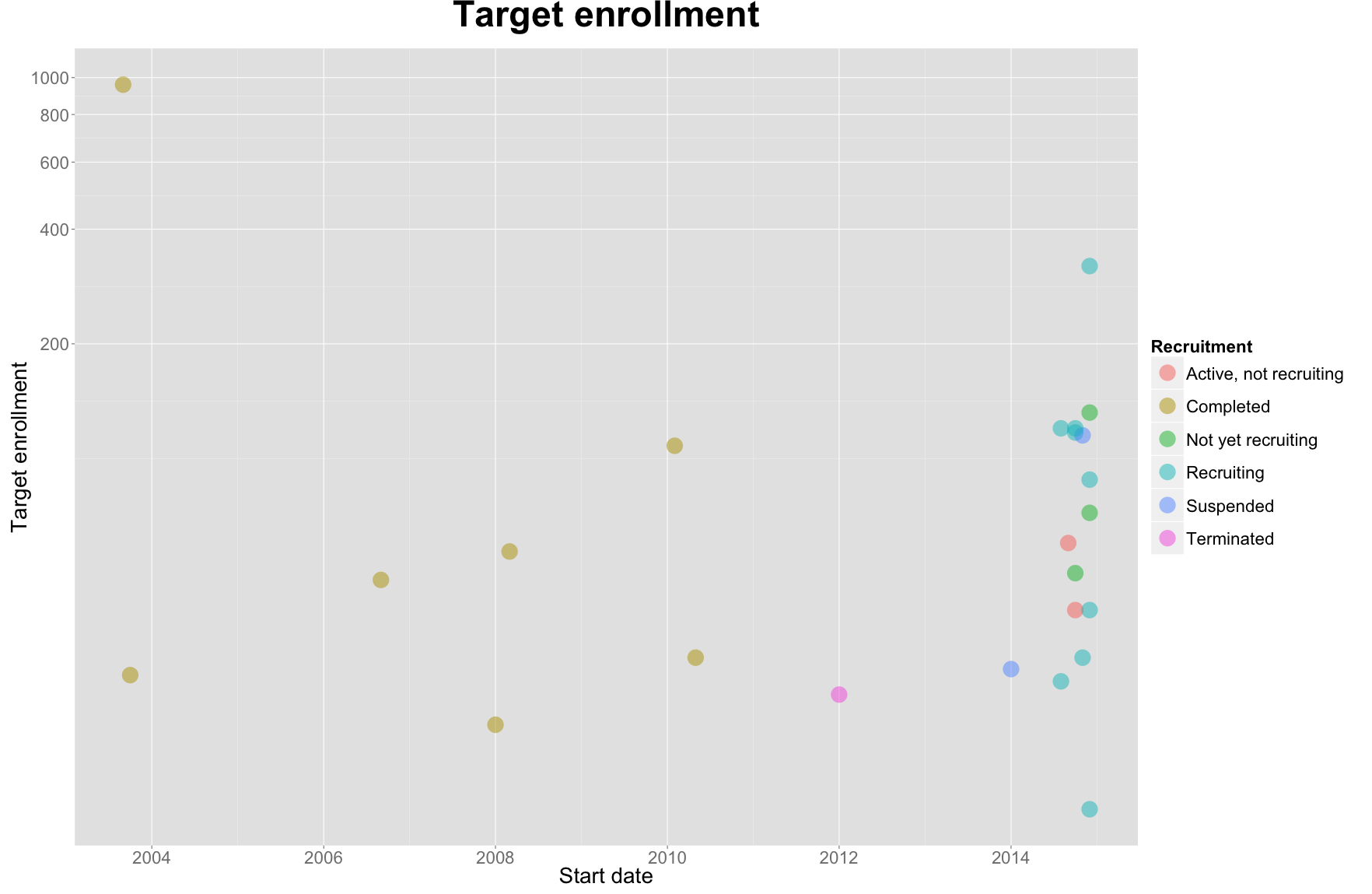
One study stands out in the plot above. In 2003, an uncommonly expansive study was launched by the US Department of Health. The National Institute of Allergy and Infectious Diseases (NIAID) in collaboration with the National Institutes of Health Clinical Center (CC) launched the study Screening of Healthy Volunteers for Clinical Trials of Investigational Vaccines to Prevent Infectious Diseases. The magnitude of the study’s enrollment target, 958, underlied its objective: funnel eligible subjects into trials for one of many “vaccines of interest” at NIAID’s Vaccine Research Center.
An overview of the types of vaccines the NIAID was interested in testing:
Of interest are vaccines for: newly identified infectious diseases such as SARS; infectious diseases of concern as possible bioweapons, such as smallpox and Ebola virus; emerging infectious diseases that are more widespread geographically than in the past, such as West Nile virus; and, for preventing diseases such as tuberculosis and malaria.
2. A majority of ebola trials have been sponsored by the US Government.
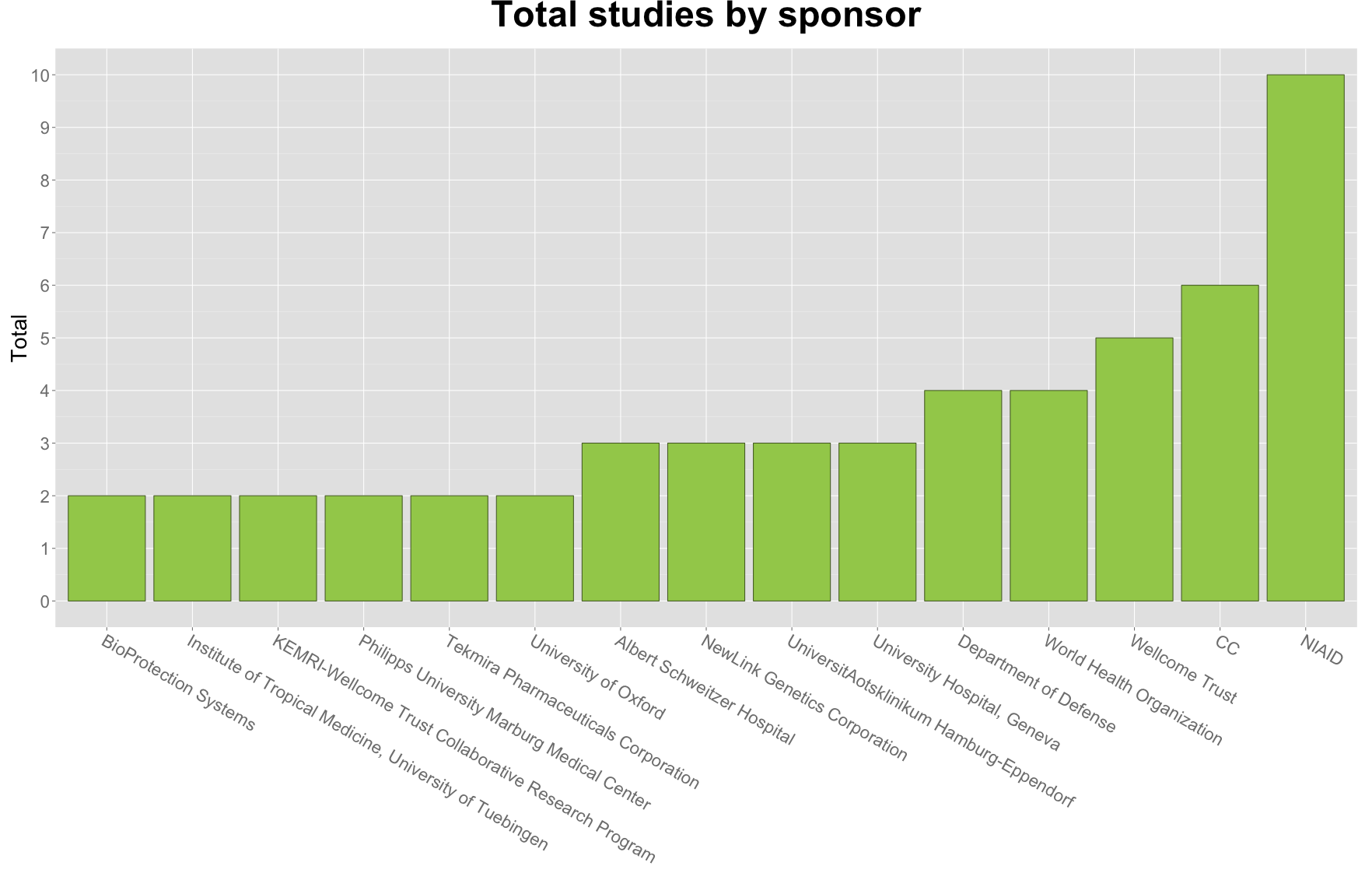
The National Institute of Allergy and Infectious Diseases (NIAID) is a sponsor in 40% of ebola trials. Also in the top 3 sponsors is the National Institutes for Health Clinical Center (CC)). NIAID and CC are both part of the US Department of Health and Human Services and together take part in two-thirds of ebola trials.
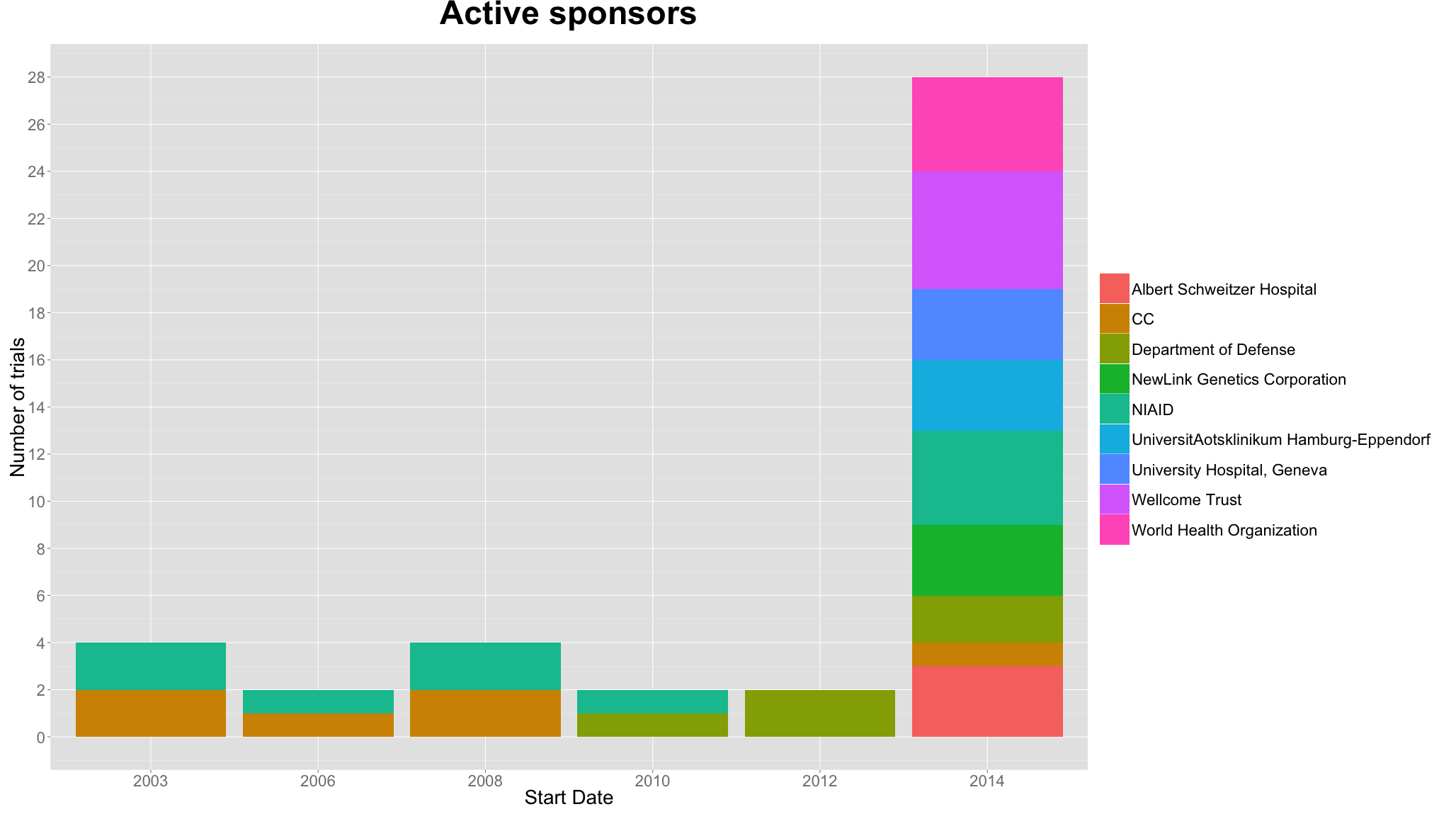
3. The most active sponsors have only become active in the past year.
Many new sponsors have become active in sponsoring ebola trials in the past year. Qualifying active sponsors as sponsors which have been involved in 3 or more trials, all active sponsors, aside from those affiliated with the US government, have become involved only in the past year. While the US Department of Defense, NIAID and CC have been sponsoring trials since 2003, in 2014, 6 other sponsors have been involved in 3 or more trials.
4. Nearly all studies are Phase I studies.
| Phase | Number of Studies |
|---|---|
| Phase 1 | 20 |
| Phase 1 / Phase 2 | 2 |
| Phase 2 | 1 |
| Phase 2 / Phase 3 | 1 |
Phase I studies focus on the safety, rather than the efficacy, of a treatment. Neither a vaccine nor a treatment for ebola is near or even on its way to FDA approval and the wider market.
